HS Diagnomics GmbH
Booth number: 59 - 04
www.hsdiagnomics.de
About us
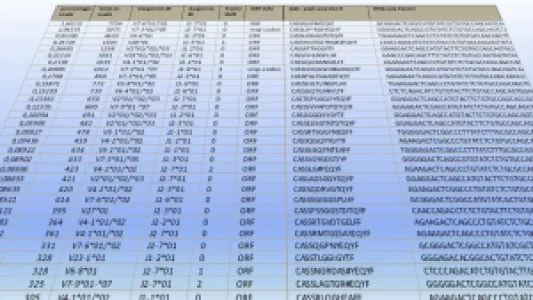
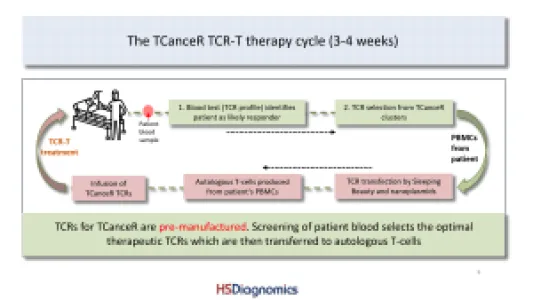
HS Diagnomics GmbH (HSD) is a Berlin-based biotech company specializing in high-resolution, quantitative T-cell receptor (TCR) profiling and tumor-specific TCR discovery for immuno-oncology and TCR-T cell therapy development. HSD combines proprietary TCRsafe technology with its TCanceR database to identify, validate, and functionally characterize tumor-specific TCRs from human samples, including fresh surgical specimens as well as FFPE material. Most important applications are personalized and off-the-shelf TCR-T therapies for solid tumors with high medical need such as lung, pancreatic, breast, and colorectal cancer. Our top TCR-T candidates are effective in a high percentage of patient tumors. Production of our therapeutic T-cells is autologous and via non-viral transfection technologies. Located in Berlin, we are working with two of the biggest clinical centers in Germany to bring our novel TCR-T therapies to solid cancer patients.
Address
Schlossstraße 110
12163 Berlin
Germany
E-mail: hennig@hsdiagnomics.de
Phone: +49 30 700145330
Internet: www.hsdiagnomics.de
Contact person:
Dr. Volker Lennerz
CSO
E-mail: lennerz@hsdiagnomics.de
Phone: +49 30 700145330
Products & Services
TCanceR-101.a: Tumor-reactivity validated in lung, pancreatic, colorectal and breast cancer samples. Approximate relevant cases per year in EU(US): Pancreatic cancer 16000 (7000), NSCLC 53000 (28000), colorectal cancer 59000 (20000). Patients will be stratified by simple blood test. Advanced pre-clinical stage.
TCanceR-101.b: Tumor-reactivity measured in lung cancer samples, predicted to be reactive also in pancreatic and colorectal cancers. Approximate patient numbers per year in EU (US): Pancreatic cancer 11000 (5000), NSCLC 37000 (20000), colorectal cancer 41000 (14000). Patients will be stratified by simple blood test. Pre-clinical stage.
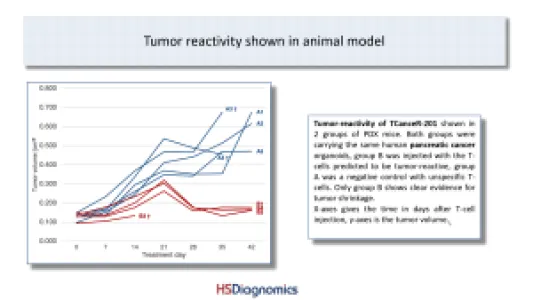
TCanceR-201: Tumor-reactivity measured in lung and pancreatic cancer samples including PDX animal model! HLA-type & mutation allows easy stratification of patients (blood test). Several hundred cases per year in Germany alone. Advanced pre-clinical stage.
TCanceR-301: Tumor-reactivity measured in liver metastases AND initial primary CRC tumors. Approximate cases per year in US/EU: 27000 patients. Explorative stage.